Na sodium Ca2 calcium K potassium Sr2 strontium. This is done for each cation individually.

Tests For Cations Edexcel Gcse Chemistry Revision Notes

Testing For Cations Sodium Hydroxide Ammonia Precipitates Compound Interest

Identification Of Ions Gases Cie Igcse Chemistry Revision Notes
To find a test for one ion that is not interfered with by another ion is nearly impossible.

Test for cations. Cations positively-charged ions and anions negatively-charged ions are formed when a metal loses electrons and a nonmetal gains them. Therefore if one has a mixture of. In Australia and New Zealand.
Circle the correct responses to complete the following statement. CHECK YOUR STRUCTURE Test your SHELX file Test your CIF file Help. Mixing If a small amount of liquid is present in a test tube it may be mixed by flicking the base of the test tube with a finger while holding the test tube lightly by the top.
If it forms a reddish-brown or black precipitate then Bi 3 Cu 2 Hg 2 or Pb 2 is present. The test site is carefully cleaned and dried then a piece of preweighed filter paper is placed over the test site and covered with parafilm to prevent evaporation. The standardised method ensures that the test specifi cations are transferable and maintainable.
Soil test reports vary from laboratory to laboratory. A lighted splint is used for the test of hydrogen. Cations 1 Cations 2 Cations 3 Cations.
Discard the contents of the test tubes in the waste container and rinse the test tubes thoroughly with deionized water. However they all report key results of pH lime test index LTI or buffer pH phosphorous and potassium. Delivery from the Dropper Withdrawal of liquid by Decant Receiving liquid from Decant Dropper Discard of all of its contents.
Next we add up the charges of each base cation. Sweat is collected for 30 minutes. A THC of either 5 nano - grams or more per milliliter of whole blood or 10 nanograms or more per milli-liter of other bodily substance.
Oxygen gas rekindles the glowing splint as it bursts into flames. It becomes easier to plan and manage the test process because the processes of test specification and execution can be split up into clearly definable blocks. Test for that ion.
Ag silver Cd 2. SILVER GROUP CATIONS Ag Pb2 Hg 2 2 Analysis of a Mixture of Cations O ne problem often faced in qualitative analysis is to test for one ion in a mixture of many ions. Briefly summarize the confirmatory test for each ion in the space provided.
EM radiation is emitted when electrons make transitions from low high to low high energy levels. Fatigue and Slurred speech. The filter paper is retrieved.
Or any trace of a drug other than cannabis illegal substance or intoxicating compound the driver will be issued a law. Click on a test tube to select it for. The test tube is place in a centrifuge for two minutes.
Understand the major difference between cations and anions in terms of Type of Element charge electrode used along with various examples. Unlike quantitative analysis which seeks to determine the quantity or amount of sample qualitative analysis is a descriptive form of analysisIn an educational setting the concentrations of the ions to be identified are approximately 001 M in an aqueous solution. The meaning of cation is the ion in an electrolyzed solution that migrates to the cathode.
Insert the tip about 05 cm below the top of the test tube and release the indicated number of drops. A glowing splint no flames burning is used to test for oxygen. SOFTWARE AND APPLICATIONS PROGRAMMERS design develop test maintain and document program code in accordance with user requirements and system and technical specifications.
Other useful measures on the report such as cation exchange capacity CEC organic matter and base saturation further define soil factors related to nutrient. Record the cations and record your observations following each. Nutrient Form used by plant Cations.
Click on the dropper bottle to place the dropper above the selected tube. Specialized collection devices may also be used. Click on Centrifuge to centrifuge ALL tubes.
2004 This version is optimized for IE 50 Netscape 46 or higher and a resolution of at least 1076 x 768 pixels For lower resolutions you should switch to the alternative version If. Otherwise if it forms a yellow precipitate then Cd 2 or Sn 4 is present. A positively charged ion.
In a flame test the element Boron emits EM radiation that is predominantly green in color. Main group cations Transition metal cations. Soil test results into yes no and maybe assists understanding the limits and benefits of using soil test results for making nutrient recommendations.
Nitrogen NH 4 Potassium K Calcium Ca2 2Magnesium Mg Manganese Mn2 2Copper Cu Zinc Zn2. Click on Heat to heat ALL tubes. These results are used to develop fertilizer recommendations.
Or if it forms a brown precipitate then Sn 2 must be present. Qualitative analysis is used to identify and separate cations and anions in a sample substance. Use a flowchart to provide the evidence for the presence of the identified cations.
Usually it is done by passing hydrogen sulfide over the test tube for detection of 1st group cations. Identifying the Cations in an Unknown Mixture Note. Sometimes the level of hydrogen cations is reported but this should not be added to your total CEC.
Report should state the identity of all cations present in the unknown. Cs cesium Zn 2. For this example the sum of.
Cations and Anions - Cations and anions are formed when a metal loses electrons and a nonmetal gains those electrons. Test for Oxygen. Rb rubidium Ba 2.
As you work document observations regarding the color of precipitates and solutions in the Figure 2 Qualitative Separation Scheme on your lab worksheet. For calcium a soil test level of 2000 ppm divided by 200 equals 100 meq100 g soil. What Are Periods Groups In The Periodic Table.
Tip of a dispensing dropper into your test solution in the test tube. If the drivers test results show a BAC of 08 or more. Most occupations in this unit group have a level of skill commensurate with a bachelor degree or higher qualification.
SoftBV Version 096 Feb. In this lab how do the metal cations become excited. Calculate collective charge from base cations.
WebMD Symptom Checker helps you find the most common medical conditions indicated by the symptoms fatigue and slurred speech including Medication reaction or side-effect Multiple sclerosis and Anemia. Or if a red orange precipitate is formed then Sb 3 is. H hydrogen Be2 beryllium Al3 aluminum Li lithium Mg2 magnesium.
Qualitative Analysis of Cations Pre-Laboratory Assignment The pre-lab assignment for Part A of the experiment is to complete the flow chart and answer the question on page 10 of this document. If your soil test report does not provide percentages you can calculate them yourself by dividing the quantity of each cation the meq figure by the CEC figure and multiplying the result by 100. Properties of Matter Chemistry FuseSchoolWhats the difference between periods and groups in the Perio.
Hydrogen gas burns with a pop sound and extinguishes the lighted splint.
Celebrate With Chemistry Test For Aqueous Cations Sodium Hydroxide
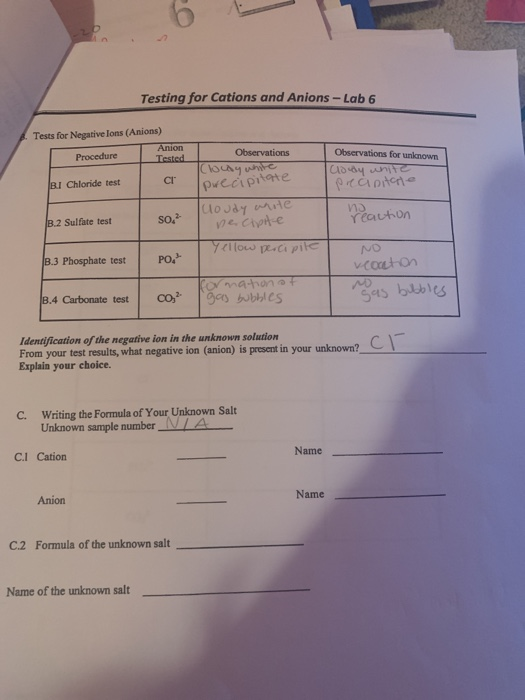
Testing For Cations And Anions Lab 6 Tests For Chegg Com
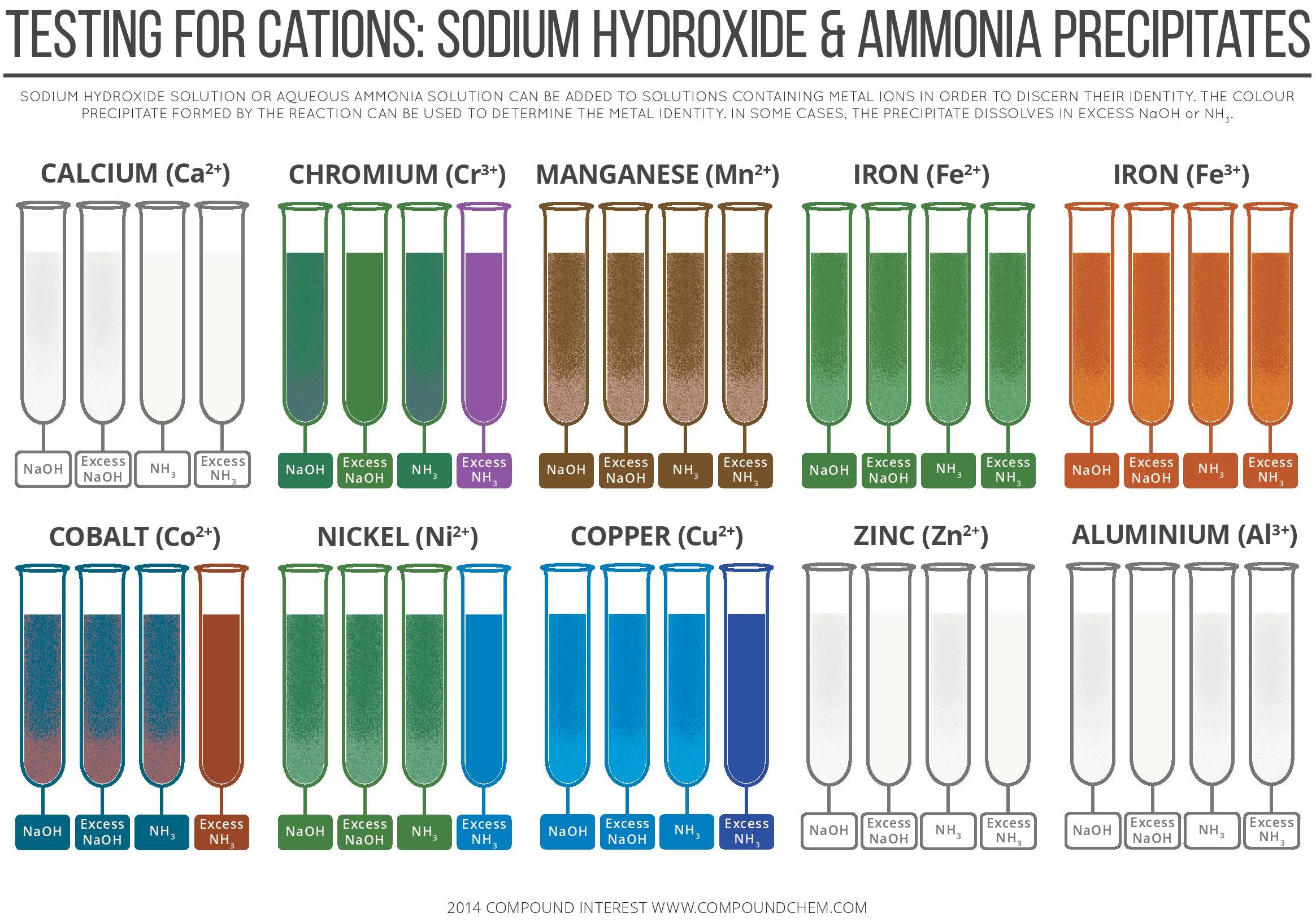
Testing For Cations By Sodium Hydroxide Ammonia Precipitates Infographic Chemistry Com Pk

Spm Chemistry A Test For Anions And Cations
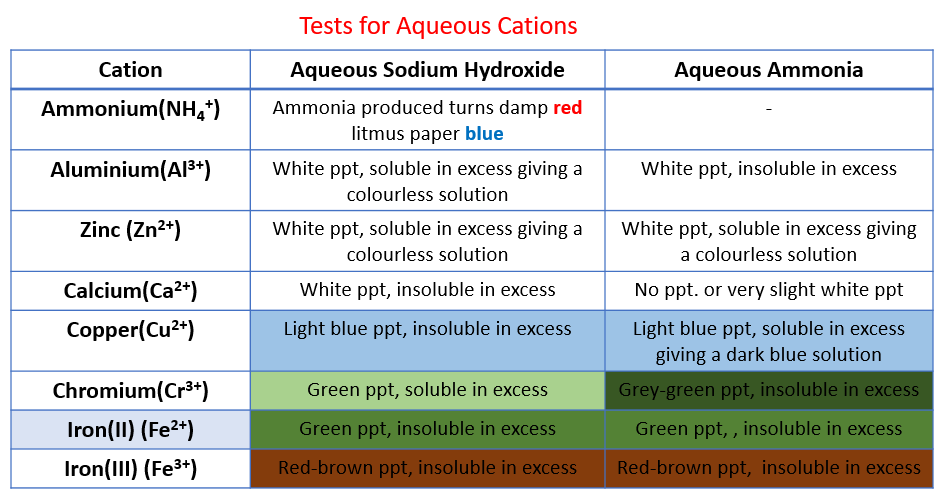
Identify Cations Solutions Examples Activities Experiment Videos

Redox Equations Titrations A Level H2 Chemistry Tuition By 10 Year Series Author

C8 4 Identification Of Ions And Gases Igcse Aid

Gcse Chemistry Chemical Tests Lesson 2 Test For Cations Aqueous Naoh Solution Youtube

