Ammonium nitrate is a nitrate salt. If youve ever had an injury while playing sport you may have used a disposable or single-use cold pack.
/93453962-56a1304e3df78cf772684199.jpg)
Cold Packs And Endothermic Reactions Example
Endothermic Reaction Read Chemistry Ck 12 Foundation

Hot Cold Packs Rachelkardishchemistry
One other example of the exothermic reaction is a cold pack.
Cold pack endothermic or exothermic. A common example is a chemical ice pack which usually contains water and a packet of ammonium chloride. This quizworksheet combo will help test your knowledge surrounding effects of endothermic and exothermic reactions. If we apply a cold pack to the place of pain after an injury then it will be an exothermic reaction.
2Na 2H 2 O 2NaOH H 2. There are many possible ingredients in an instant cold pack but they often contain solid ammonium nitrate and water. The reaction was exothermic meaning it released heat and increased the temperature of its.
Powered by FlexBook textbook Platform CK-12 Foundation 2021. These are called exothermic exo meaning outside thermic. The dissolving reaction is endothermic - requires heat.
Used in heat packs heat packs used by sports people to relax their muscles. A salt such as ammonium nitrate is dissolved in water after a sharp blow breaks the containers for each. In this hands-on activity students use a coffee cup calorimeter to measure the heat of solution of a chemical salt using 3 different masses and then design their own hot andor cold pack.
When chemical reactions or processes occur there is always an exchange of energy. Endothermic exothermic neither endothermic nor exothermic both endothermic and exothermic. An instant cold pack.
Lets see how it works. 4Fe s 3O 2 g 2Fe 2 O 3. The cold pack has two bags - an inner bag and an outer bag.
How about an instant cold pack to treat an injury. In the Feel the Heat Gizmo create your own hot and cold packs using various salts dissolved in water and different bag materials. Chemical Reaction Formula Examples.
Have you ever noticed how cold your slime gets. After an exothermic reaction more energy has been released to the surroundings than was absorbed to initiate and maintain the reactionAn example would be the burning of a candle wherein the sum of calories produced by combustion found by. This kind of endothermic process is used in instant cold packs.
The melting of the ice cube is an example of an endothermic reaction heat is being absorbed from the surroundings. Heat is abbreviated as. A salt such as ammonium nitrate is dissolved in water after a sharp blow breaks the containers for each.
This is the reaction that happens inside of a cold pack found in emergency kits. This means that the reaction taking place is endothermic or exothermic 10. A primary feature of any calorimeter must be.
Learn about exothermic and endothermic processes and how energy is absorbed or. This is an endothermic reaction at play. When someone gets hurt playing sports often times a cold pack is used for the injury.
Sodium metal Na reacts with water H 2 O to form sodium hydroxide NaOH and hydrogen gas H 2. Introduce the terms endothermic and exothermic. Endothermic or exothermicThe Greek root therm means temperature or heat which gives us a clue about all reactions.
The result is an endothermic reaction that cools the pack down and it can be used to stop or reduce swelling. Slime is an endothermic reaction as opposed to an exothermic reaction. A discussion of chemical hot and cold packs can really warm up a classroom lesson on thermochemistry.
When the cold pack is squeezed the inner bag of water breaks and the water mixes with the chemicals. The heat pack uses the reaction of oxidation of iron iron and oxygen reacting which is exothermic. When the bag is.
The majority of chemicals used in these packs are non-toxic and pose no threat to the environment. CH 4 2O 2 CO 2 2H 2 O. Endothermic Fire Pit is more fuel-efficient than the Endothermic Fire.
The chemicals in hot packs combine to cause exothermic reactions while those in cold packs cause endothermic reactions. In the bag there are two components. An instant cold pack is the perfect example of an endothermic reaction.
When two chemicals are added together. Two types of chemical reactions. Hot and cold packs contain mixtures of chemicals that react together to create heat and cold 1.
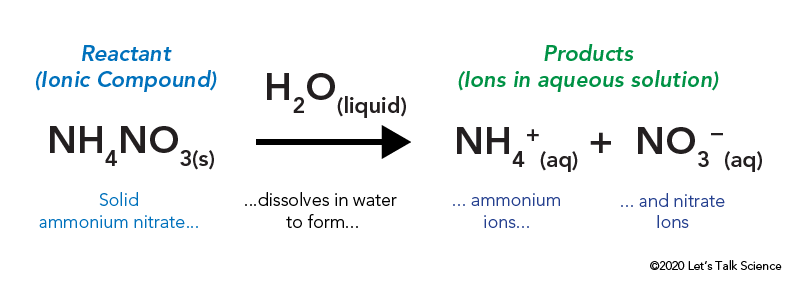
Dissolving the substance from the hot pack will cause the temperature to increase to over 40 C exothermic. In this case you break a barrier in the cold pack which allows ammonium nitrate NH 4 NO 3s to dissolve in. The surrounding temperature go down.
CH4 and O2 are the reactants while CO 2 and H 2 O are the products. Some of these reactions or processes give off energy as heat. A damp mixture of iron filings with salt and charcoal in a perforated bag.
The cold pack is activated by breaking the barrier separating the water and ammonium chloride allowing them to mix. There is energy exchangeEndo means within while exo means outside so these types of reactions are opposite. If a reaction inside a calorimeter is endothermic we should see.
Endothermic reactions are those which absorb heat during the reaction. Methane CH 4 and oxygen O 2 react to produce carbon dioxide CO 2 and water H 2 O. Both the cold packs and the hot packs use chemistry to change their temperature.
Water and ammonium nitrate. Exothermic and endothermic describe two types of chemical reactions or systems found in nature as follows. Endothermic or exothermic 9.
NH4NO3s NH4NO3aq Is the reaction endothermic or exothermic. Each fuel item burns as long as they would in a Fire Pit which is twice as long as the burn time in a Campfire or Endothermic Fire. The cold pack takes up more energy than it gives off and it gets very _____ to the touch.
Now lets go back to our instant cold pack. This process is also known as an exothermic reaction. Reactions that absorb heat from the environment are called endothermic reactions.
The dissolving reaction is endothermic - requires heat. Therefore the heat is. Squeezing the cold pack bursts a small inner bag allowing two chemicals to mix.
The use of first-aid instant cold packs is an application of this solubility principle. Slime activators borax sodium borate and boric acid change the position of these molecules in a process called cross-linking. Dissolving the substance from the cold pack will cause the temperature to decrease to less than 10 C endothermic.
Cold packs to heal an injury. Every chemical reaction that exists is one of two things. If youve ever sprained your ankle or fell from your bike youve probably reached for an instant cold pack.
A cold pack utilizes what kind of reaction to cool your. Using exothermic and endothermic reactions. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical or mixture of chemicals that result in an endothermic reaction when dissolved in water.
How do thermodynamics work in a cold pack. An endothermic reaction absorbs energy heat instead of giving off energy heat. The Endothermic Fire Pit is the permanent version of the Endothermic Fire.
The use of first-aid instant cold packs is an application of this solubility principle. It requires an Alchemy Engine to prototype and costs 2 Nitre 4 Cut Stone and 2 Electrical Doodads to craft.

Exothermic And Endothermic Processes Ck 12 Foundation
The Cold Pack A Chilly Example Of An Endothermic Reaction Let S Talk Science
Chemistry Phenomenon Endothermic Exothermic Reactions In Everyday Life

Endothermic Exothermic Reactions Ppt Download

Lecture 17 1 Endothermic Vs Exothermic
Solved Determine Whether Each Described Process Is Chegg Com

Chemistry Thermochemistry 36 Of 37 The Cold Pack Youtube
The Cold Pack A Chilly Example Of An Endothermic Reaction Let S Talk Science

